DIMENZIJE
| Model | Maksimalni kapacitet (l/min) |
Max tlak (H) |
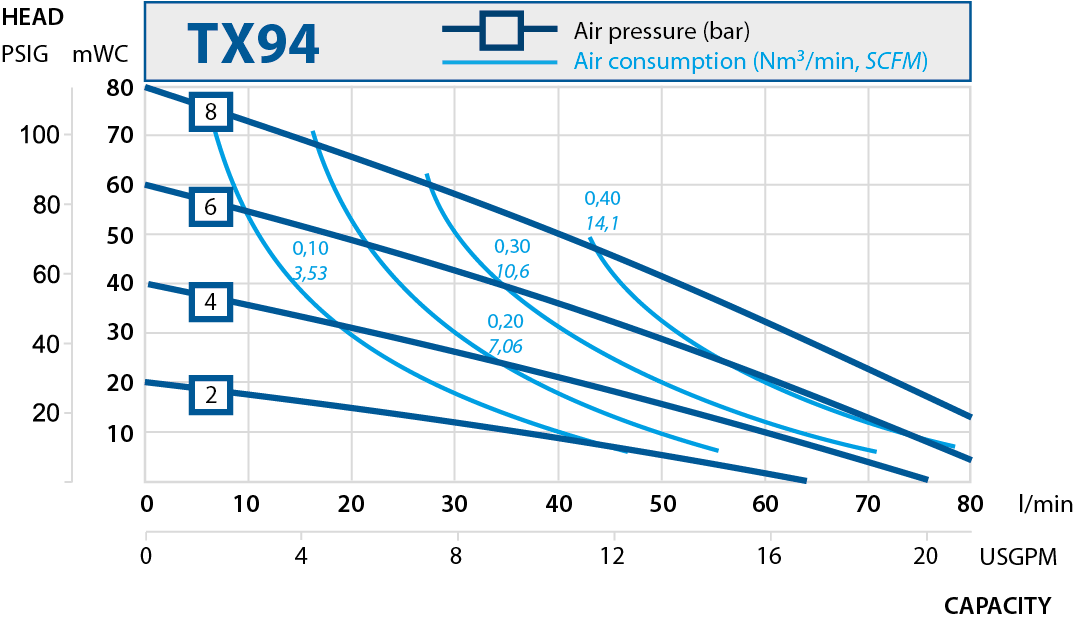
| TX94 | 94 | 8 |
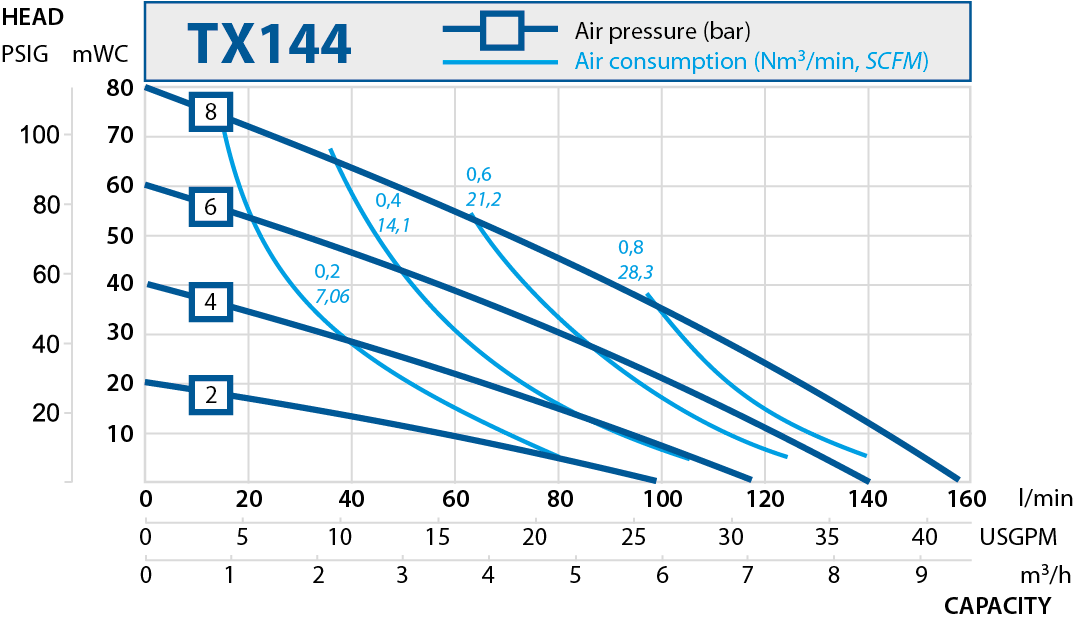
| TX144 | 144 | 8 |
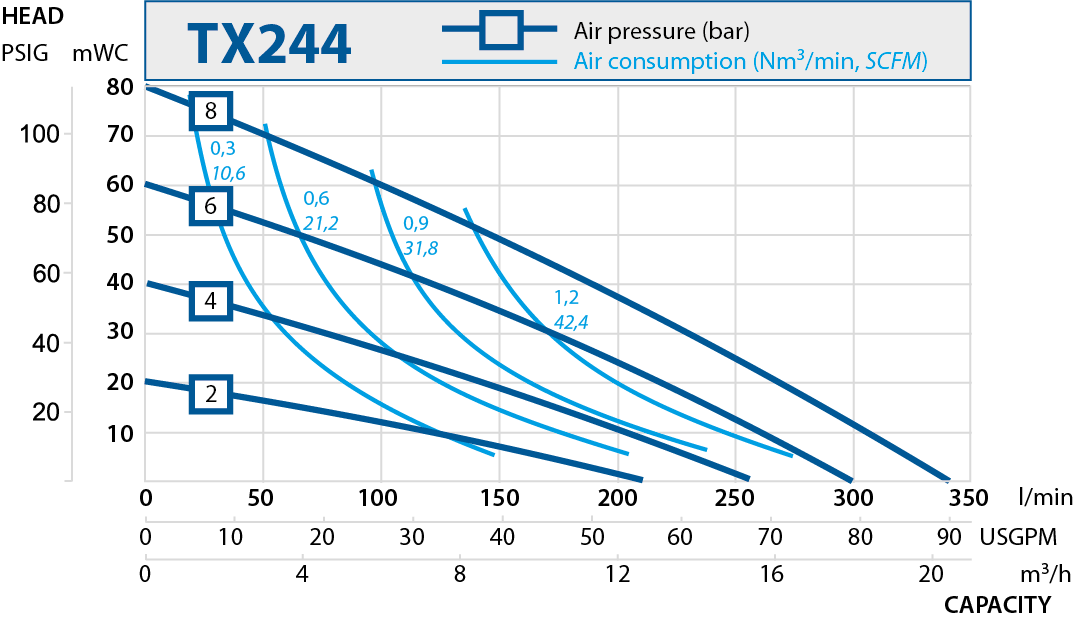
| TX244 | 270 | 8 |
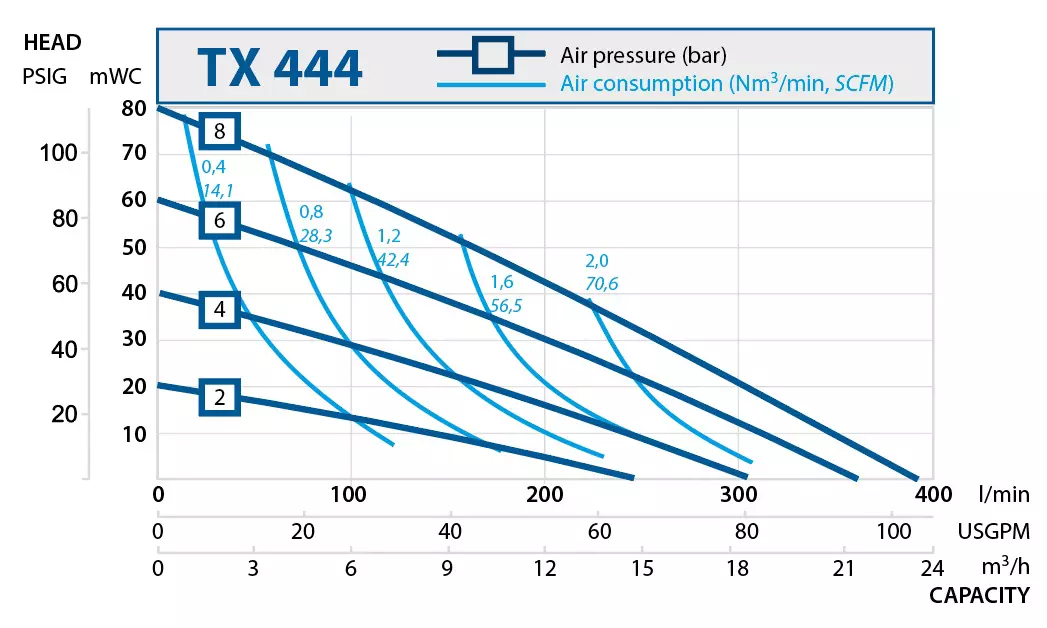
| TX444 | 360 | 8 |
EHEDG CERTIFIKAT

Certifikat EHEDG (European Hygienic Engineering & Design Group) vaše je jamstvo da je dizajn u skladu s higijenskim smjernicama. Nadalje, pumpa je testirana na mogućnost čišćenja, što znači da se bakterije ne razvijaju u pumpi nakon postupka čišćenja i pražnjenja.
Održavanje vašeg procesa čistim
Tapflo pumpe iz aseptične serije dizajnirane su za rad u farmaceutskoj, biotehnološkoj i prehrambenoj industriji gdje je ključan čist proces
Serija Tapflo aseptičnih pumpi EHEDG certificirana, ima materijale odobrene od strane FDA i USP VI i u skladu je s ATEX direktivom 2014/34/EU.
TIPIČNE PRIMJENE
- Hrana i mliječni proizvodi: juha, vrhnje, sirup, mliječni proizvodi, arome, alkohol, čokolada, pasta
- Farmaceutika i kozmetika: krema, pasta, alkohol i filtracijski gel
ZNAČAJKE I PREDNOSTI
- Nema rasta bakterija
- Jednostavno čišćenje i pražnjenje
- Nježno pumpanje
- Širok raspon vrsta priključaka
- Higijenske površine
- Nema curenja
- Fleksibilna instalacija
- Pouzdana u radu
- Ekološki prihvatljivo
- Higijenske dijafragme


 Mobile SI:
Mobile SI: